01/01/2024 | ORP | 12 MINUTE READ
Understanding the Crucial Role of ORP and pH in Reverse Osmosis Systems

In the intricate world of water purification, where the demand for pure water is paramount, the reverse osmosis (RO) process stands as a stalwart, ensuring the removal of impurities and the delivery of high-quality water. However, to navigate the complexities of RO systems effectively, one must delve into the nuances of two pivotal parameters: Oxidation Reduction Potential (ORP) and pH.
As we embark on this exploration, we will unravel the fundamentals of reverse osmosis, understanding its challenges, and the critical need for precise measurements. Join us on a journey through the significance of ORP in evaluating membrane health, the influence of pH on membrane life and scaling potential, and the real-world applications that underscore the importance of these measurements in maintaining water purity. Let’s unlock the power of precision and unveil the secrets that contribute to the success of reverse osmosis systems.

The Fundamentals of Reverse Osmosis (RO) Systems
Reverse osmosis (RO) is a cornerstone in the realm of water purification, offering a sophisticated process to ensure water quality in various applications. To comprehend the significance of Oxidation Reduction Potential (ORP) and pH in this context, a foundational understanding of RO systems is essential.
In the initial phases, local water sources, often derived from rivers or lakes, undergo mandatory pre-treatment and purification processes before embarking on the RO journey. This preparatory stage is essential to optimize the efficiency of the subsequent purification steps.
Common Challenges in RO Systems: Navigating through the intricacies of RO systems reveals common challenges that demand attention. Preliminary purification involves essential stages such as filtration, clarification, and softening, each contributing to the removal of impurities. Downstream processes introduce advanced techniques like the two-pass RO system and demineralization operations, amplifying the purification process. The integration of these stages underscores the comprehensive nature of water treatment in RO systems.
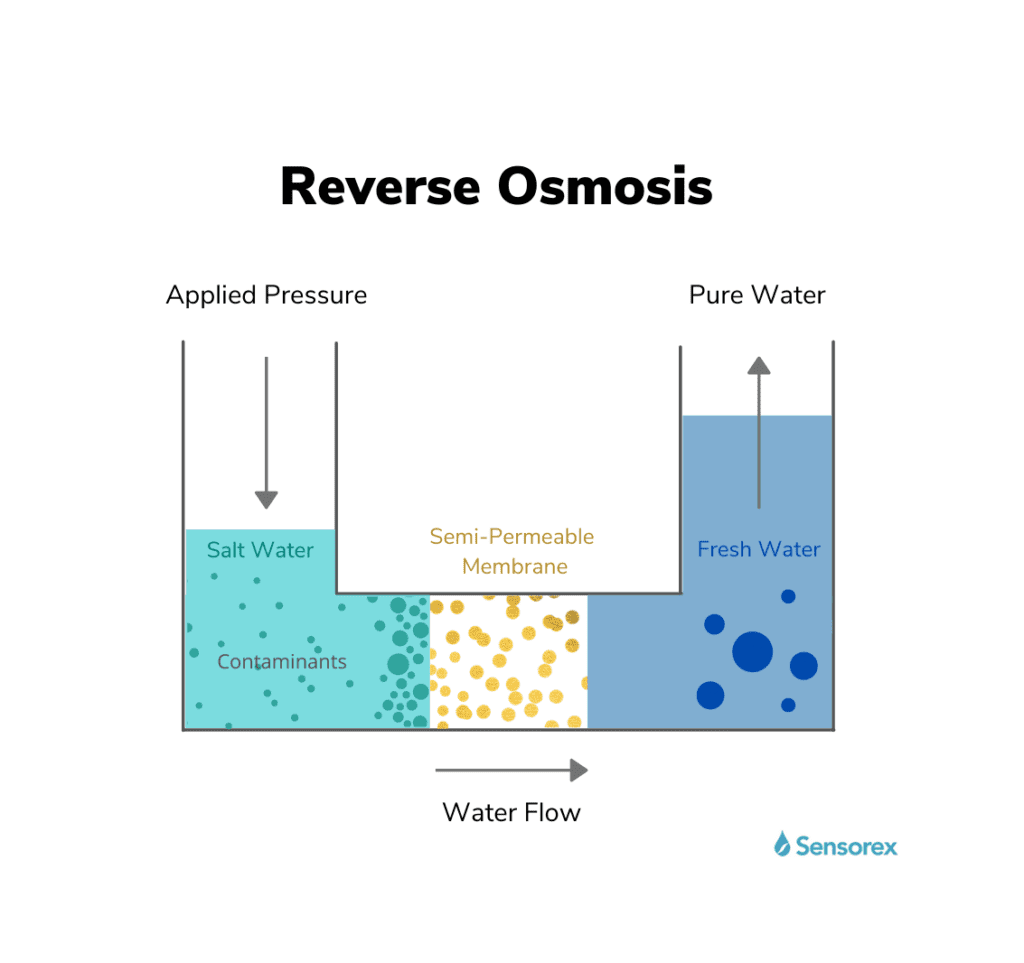
Osmosis and the Role of Pressure: Fundamental to the RO process is the phenomenon of osmosis, where fluids naturally move through semi-permeable membranes from less concentrated solutions to more concentrated ones. This principle guides the initial stages of purification. In the context of RO, the application of pressure becomes a crucial element. By exerting pressure on the side with the concentrated solution, water molecules are compelled to traverse the semi-permeable membrane, ultimately reaching the side with pure water.
Impurities and Membrane Efficiency: Semi-permeable membranes serve as guardians against the passage of dissolved impurities. The efficiency of these membranes is not only influenced by the nature of impurities but also by factors such as age, cleanliness, and the magnitude of applied pressure. The utilization of a cross-flow filtration mode enhances efficiency by diverting some feed water to cleanse away rejected impurities. This process results in the production of permeate and concentrate streams, each contributing to the overall quality of the treated water.
RO System Operation and Maintenance: RO systems are designed for automatic operation, incorporating routine preventative and corrective maintenance procedures. This automated approach ensures the consistent performance of the system. Common issues, including membrane fouling and suboptimal flow rates, pose challenges that can impact throughput capacity and system longevity. Regular maintenance emerges as a critical component, preventing membrane failure and mitigating the risk of overburdening downstream purification systems. The commitment to regular upkeep is paramount for sustaining the efficiency and reliability of RO systems in the long run.
In grasping these fundamentals, we lay the groundwork for a deeper exploration into how ORP and pH measurements play pivotal roles in optimizing the efficiency and longevity of reverse osmosis systems.
The Role of Oxidation Reduction Potential (ORP) in RO Systems
In the intricate dance of water purification through reverse osmosis (RO) systems, Oxidation Reduction Potential (ORP) emerges as a critical parameter, akin to a guardian of membrane health and system efficiency. Understanding the role of ORP in the context of RO systems unveils its significance in safeguarding the integrity of the purification process.
In the landscape of reverse osmosis (RO), Oxidation Reduction Potential (ORP) emerges as a crucial metric, offering insights into the activity of oxidizers within the system. ORP, shorthand for Oxidation Reduction Potential, serves as a quantitative measure that gauges the potential for oxidation and reduction reactions in the RO environment.
Membrane Susceptibility to Oxidizers: The membranes employed in RO systems are inherently vulnerable to attack by various oxidizers, including chlorine, bromine, ozone, and hydrogen peroxide. These substances pose a potential threat to the integrity and longevity of the RO membranes, necessitating a vigilant approach to their detection and control.
Detecting Oxidizer Activity: The detection of oxidizer activity is paramount for ensuring the health of RO membranes. A heightened ORP reading serves as a clear indicator of the presence of active oxidizers in the system. This reading goes beyond traditional chemical residual measurements, providing valuable information about the ability and speed of oxidation processes within the RO environment.
Utilizing ORP to Optimize Treatment: ORP measurements offer a dynamic tool for optimizing treatment processes in RO systems. The overfeed of sodium bisulfite, employed to reduce chlorine levels, can be effectively monitored through ORP readings. An ORP reading falling below 200 mV signals a reducing condition, prompting attention to prevent extra costs and potential environmental discharge issues associated with overfeeding.
Application of SE 565 ORP Electrode: The S8000CD-ORP and S272CD-ORP electrodes emerges as invaluable instruments for precise and reliable ORP monitoring. Beyond its accuracy, the design of this electrode contributes to an extended sensor life, reinforcing its role as a dependable means to control the addition of sodium bisulfite and prevent potential overfeeding scenarios.
Beyond Membrane Protection: Real-world Implications: ORP transcends its role as a protector of membranes; it serves as a key indicator for necessary pretreatment measures. This proactive approach helps prevent membrane fouling, a common challenge in RO systems. Additionally, a low ORP reading may signify the presence of biological activity, offering a real-world implication that demands attention to avoid potential fouling of membranes and maintain system efficiency.
Understanding the nuances of ORP in RO systems not only ensures the protection of membranes from oxidizer-induced damage but also empowers operators to make informed decisions, optimize treatment processes, and ultimately, enhance the overall efficiency of reverse osmosis systems.

Ensuring Water Quality with pH/ORP Measurements
In the realm of reverse osmosis (RO) systems, where the quest for water purity is paramount, the synergy between pH and Oxidation Reduction Potential (ORP) emerges as a dynamic duo, ensuring not only the health of the membranes but the overall water quality. Let’s delve into how pH and ORP measurements play a pivotal role in safeguarding the successful operation of RO systems.
- Sensitive RO Membranes and pH Control: Adding nuance to this delicate balance is the sensitivity exhibited by certain RO membranes to the pH of the feedwater. In response to this sensitivity, an upstream pH sensor assumes the role of a crucial feedback mechanism. This sensor provides real-time signals, empowering operators to exercise precise control over the dosing of acidic or basic reagents, ensuring that the pH remains within the optimal range of 5 to 8 pH.
- ORP: In parallel to pH, the Oxidation Reduction Potential (ORP) steps into the limelight as a dynamic indicator of system activity. Measuring the potential for oxidation and reduction reactions, ORP serves as a key parameter in gauging the activity of oxidizers that pose a potential threat to RO membranes. High ORP readings signal the need for pretreatment, while low readings may signify biological activity, indicating a potential risk of fouling.
- The Confluence of pH and ORP: The synergy between pH and ORP measurements unfolds as a real-time monitoring mechanism, providing operators with invaluable insights into the condition of RO membranes. This dynamic duo grants enhanced control over the addition of chemicals, leading to cost reduction and preventing the risk of overdosing, thereby optimizing the overall efficiency of the RO system.
- Balancing Act for Optimal Operation: Completing this intricate ballet of measurements is the incorporation of conductivity measurements at both the inlet and outlet of the RO unit. These measurements, working in tandem with pH and ORP monitoring, ensure the effective removal of total dissolved solids. As part of routine measurements, they stand as a proactive approach, acting as guardians of membrane health, preventing fouling, and contributing to the optimization of the entire RO system’s performance.
As we navigate the intricate landscape of pH and ORP measurements, we unveil a precision approach that not only guards RO membranes against potential threats but also elevates the entire water purification process to new levels of efficiency and reliability.
The Impact of ORP and pH on High-Purity Water Processing
In the pursuit of high-purity water, where precision is paramount, the interplay between Oxidation Reduction Potential (ORP) and pH becomes a defining factor in the success of reverse osmosis (RO) systems. As we delve into the intricacies of high-purity water processing, we uncover how ORP and pH wield their influence, steering the course toward optimal performance.
The pursuit of achieving high-purity permeate unveils a delicate equilibrium shaped by minor feedwater constituents, notably alkalinity and ammonia. These elements, seemingly minor yet impactful, necessitate vigilant monitoring for the seamless production of high-purity water. Variations in alkalinity and ammonia levels demand meticulous attention, underscoring the importance of a nuanced approach in water treatment processes.
In the intricate ballet of high-purity water production, the calculation of % rejection takes center stage, guided by conductivity measurements at both the inlet and outlet. This dynamic duo, working in tandem, provides a quantitative measure of dissolved solids removal efficiency. The formula [% rejection = [1 – (cell2)/(cell1)] x 100] unveils the efficiency spectrum, a vital metric in the meticulous dance of achieving water purity.
As the performance reaches its crescendo, a final conductivity measurement post the second stage assumes the role of a definitive quality check. This step, akin to the final notes in a symphony, determines the absolute quality of the outlet water. Its significance lies in ensuring that the high-purity water aligns with the stringent standards mandated for specific applications, marking the culmination of the water treatment symphony.
The delicate equation of high-purity water production encounters a nuanced challenge with the introduction of ammonia, whether from municipal chlorination or organic contamination. Understanding the molecular and ionic forms of ammonia that traverse the membrane system becomes crucial in maintaining the delicate balance required for water purity.
In the presence of ammonia, the intricate dynamics between pH and ORP come to life. This precision dance provides valuable insights into the complex scenario at hand. Ammonium hydroxide’s lower conductivity compared to ammonium carbonate and the shift in pH due to CO2 absorption underscore the nuanced interactions that demand precision in water treatment.
The efficiency determinants in high-purity water production find their measure in the % rejection spectrum. Gauging the overall efficiency of dissolved solids removal becomes a crucial aspect, essential for achieving the desired water purity. In applications of this nature, the typical range for % rejection falls between 80% and 100%, marking the standards that define success in water treatment symphonies.
As we navigate the waters of high-purity water processing, the dynamic interplay between ORP, pH, and conductivity emerges as a symphony of precision. Balancing the delicate equations, understanding dependencies, and leveraging real-time measurements elevate the efficiency of RO systems, ensuring the delivery of water that meets the most stringent standards for diverse applications.

Conclusion
In the intricate dance of water purification through reverse osmosis (RO) systems, the harmonious interplay of Oxidation Reduction Potential (ORP) and pH emerges as the linchpin for success. As we conclude our journey into the realms of precision monitoring in RO systems, it becomes evident that these measurements are not merely metrics; they are guardians of water quality, sentinels ensuring the longevity and efficiency of the entire purification process.
In this symphony of water treatment, ORP assumes the role of a vigilant protector. From safeguarding membranes against oxidizers to detecting overfeeds and signaling potential biological activity, ORP stands as a watchful guardian of RO systems. Alongside ORP, pH becomes the discerning eye in the quest for purity. With its ability to predict membrane life, control scaling potential, and influence the efficiency of high-purity water production, pH adds a layer of precision to the symphony.
The real-time insights provided by pH and ORP measurements empower operators to make informed decisions, optimizing chemical dosing, preventing overfeeds, and ensuring the system operates within the optimal pH range. This dynamic monitoring is complemented by conductivity measurements, serving as efficiency metrics at both the inlet and outlet. Together, they provide a proactive approach to maintain membrane health and prevent fouling, ensuring operational excellence.
In the realm of high-purity water processing, the pH dependency of two-pass RO systems and the careful consideration of minor feedwater constituents become crucial elements in achieving the desired purity. The calculation of % rejection through conductivity measurements quantifies the efficiency of dissolved solids removal, providing a quantitative measure for quality assurance.
Understanding the delicate equilibrium impacted by ammonia presence and the dynamic shifts in pH/ORP dynamics highlights the need for precision in handling complex scenarios. In this intricate navigation, the final check comes in the form of a conclusive step – a final conductivity measurement. This ensures that high-purity water meets stringent standards, marking the completion of the journey towards water purity.
In the realm of reverse osmosis, where water purity is paramount, the orchestration of pH and ORP measurements becomes more than a science; it becomes an art form, shaping the symphony that defines the success of the entire water purification process.
Posted by Joshua Samp on January 1, 2024
Sensorex is a global leader in the design and manufacture of quality sensors for water quality and process applications. The company offers more than 2000 sensor packages for pH, ORP, conductivity, dissolved oxygen, free chlorine, chlorine dioxide, UV transmittance and other specialty measurements, as well as a full line of sensor accessories and transmitters. Its expert technical support engineers solve analytical sensor challenges with custom designs and off the shelf products.




